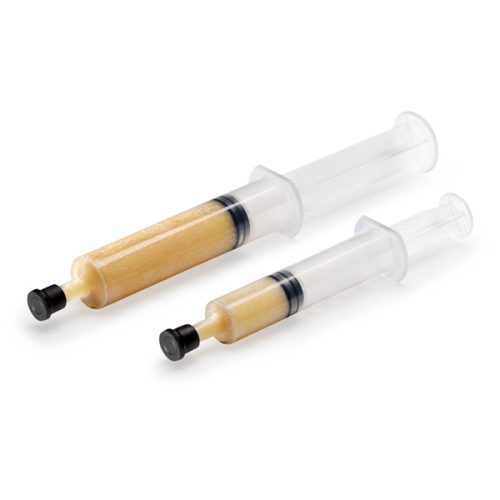
Accell Connexus is highly inductive 2nd Generation DBM with a DBM content of close to 70%. It is sold in a syringe form and also contains the unique RPM carrier which is also an inductive material, giving it exceptional handling characteristics. Accell Connexus should be mixed with any Allograft, Autograft or a synthetic scaffold to provide the ideal recipe for fusion – of conductivity and inductivity.
Accell Technology features a patented process that produces the Accell bone matrix (ABM) which is an open-structured, dispersed form of Dynamic Bone Matrix (DBM). The ABM study uses a skeletally mature sheep model with cylindrical 5mm metatarsal defect which is created in tibial diaphysis and which shows excellent results after 4 weeks. This product benefits from validated osteoinductive potential, is mouldable and easy to pack into defects making it ready to use.
Case studies have shown positive results when used in:
- Spine: Intertransverse process fusion
- Hand: Cystic scaphoid nonunion
- Foot & Ankle: - Tibial osteotomy - Hindfoot and ankle nonunions