Please feel free to browse our extensive range of Spinal Instruments, Implants and Bone products available to both the NHS and Private Hospital Sector for direct purchase or on a hire basis. A few of our brands include Joimax, Medicon, Spinal Kinetics, Implanet, Edge and Allograft – all in which comply with current traceability legislation and GS1 code practise.
-
 We supply a wide range of articulated joint repair ligaments and tendons including:All of our tendons and ligaments undergo a rigorous Supercritical CO2 Sterilisation Process which is explained in further detail below.
We supply a wide range of articulated joint repair ligaments and tendons including:All of our tendons and ligaments undergo a rigorous Supercritical CO2 Sterilisation Process which is explained in further detail below.- Patella Tendon Whole
- Patella Tendon Hemi
- Pre-Shaped Patella Tendon
- Achilles Tendon w/Bone Block
- Achilles Tendon w/out Bone Block
- Pre-Shaped Achilles Tendon w/Bone Block
- Gracilis Tendon
- Semitendinosus Tendon
- Semitendinosus-Gracilis Tendon
- Tibialis Tendon Anterior
- Tibialis Tendon Posterior
- Peroneus Longus
-
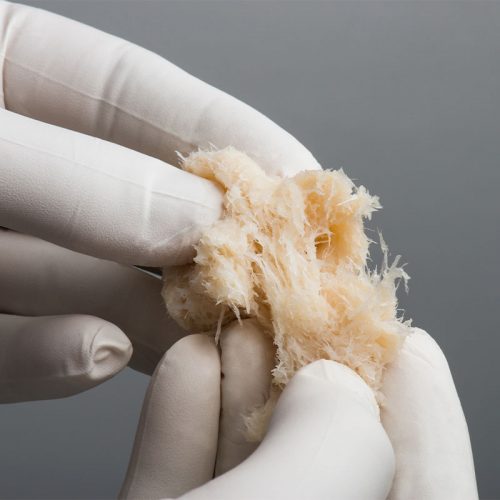
 The Seaspine Strand is made from 100% demineralised bone fibres and is designed to encourage bone growth. Developed through a disciplined R&D procedure, the Strand has evaluated a variety of fibre geometries to deliver osteoinductivity, osteoconductivity, intraoperative handling, and controlled expansion. The Seaspine Strand Plus is powered by Accell® Bone Matrix (ABM) which provides both immediate and sustained release of growth factors. Key Features for the Strand:
The Seaspine Strand is made from 100% demineralised bone fibres and is designed to encourage bone growth. Developed through a disciplined R&D procedure, the Strand has evaluated a variety of fibre geometries to deliver osteoinductivity, osteoconductivity, intraoperative handling, and controlled expansion. The Seaspine Strand Plus is powered by Accell® Bone Matrix (ABM) which provides both immediate and sustained release of growth factors. Key Features for the Strand:- 100% Demineralised Bone Fibres
- Expands to Fill Gaps
- Cellular Highways
- Simplicity of Hydration
- 100% Demineralised Bone Fibres with ABM
- 100% Demineralised Bone Fibres
- Expands to Fill Gaps
- Cellular Highways
- Simplicity of Hydration
-

For painful bone tumours
The OsteoCoolTM RF Ablation System is cooled radiofrequency (RF) ablation technology. It offers simultaneous, dual-probe capabilities for treating painful bone tumours. Product DetailsThe OsteoCool RF Ablation system is cooled radiofrequency (RF) ablation technology. It offers simultaneous, dual-probe capabilities for treating painful bone tumours.
Coaxial, bipolar technology delivers RF energy to the site, and automatically moderates power to keep RF heating within the desired treatment range. This reduces risk of potential thermal damage to adjacent tissue.1
The active tip of the ablation probe is internally cooled with circulating water. RF energy heats the tissue while circulating water moderates the temperature close to the active tip. This combination:
- Creates large volume lesions without excessive heating at the active tip
- Minimises potential for char
The OsteoCool RF ablation probes are sterile and intended for single use.
1 Based on internal testing. Data on file.
-
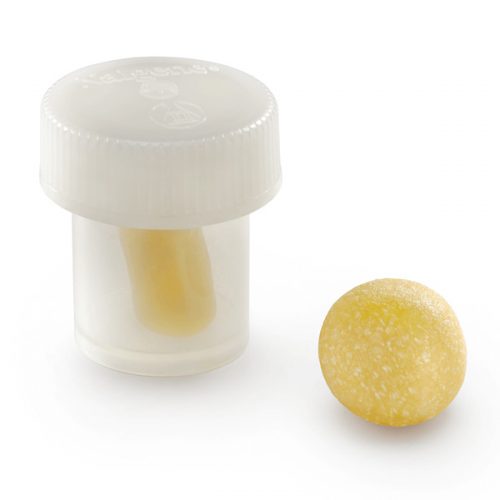 Orthoblast II is a powerful choice of bone grafting and it is what is termed a “complete” bone graft, offering both conductivity and inductivity to the user Surgeon. It is available as a Putty in a tub where the conductive bone graft granules mixed in with the DBM Putty are larger given the method of delivery. This is a crunchy graft when handled by the Surgeon. The other method of delivery with this product is via a syringe, where the conductive granules are slightly smaller simply because they need to go through the tip of the syringe. This is no less a complete bone graft however, it is simply another method of delivery for the Surgeon, and contains demineralized bone matrix (DBM) to stimulate and regenerate new bone growth. It is a highly inductive material supplied in syringe form (smaller particulate bone granules) and in tub form (larger particulate bone granules). OrthoBlast® II therefore, combines demineralized allograft bone with cancellous bone in a reverse phase medium (RPM) carrier, to provide an osteoconductive & Inductive allograft with ideal handling qualities. OrthoBlast II has enjoyed clinical success in a variety of surgical applications including periarticular defects and long-bone defects. An independent study of two DBM allografts, in cases of metaphyseal and periarticular fractures, concluded that the OrthoBlast II success rate was over 30% higher than the alternative allograft product. In another independent study of the use of DBM allograft products in ankle/hindfoot fusion, 14% of patients with a glycerol based allograft developed a non-union, versus only 8% for the OrthoBlast patients. We offer a 24 hour delivery service for this product.
Orthoblast II is a powerful choice of bone grafting and it is what is termed a “complete” bone graft, offering both conductivity and inductivity to the user Surgeon. It is available as a Putty in a tub where the conductive bone graft granules mixed in with the DBM Putty are larger given the method of delivery. This is a crunchy graft when handled by the Surgeon. The other method of delivery with this product is via a syringe, where the conductive granules are slightly smaller simply because they need to go through the tip of the syringe. This is no less a complete bone graft however, it is simply another method of delivery for the Surgeon, and contains demineralized bone matrix (DBM) to stimulate and regenerate new bone growth. It is a highly inductive material supplied in syringe form (smaller particulate bone granules) and in tub form (larger particulate bone granules). OrthoBlast® II therefore, combines demineralized allograft bone with cancellous bone in a reverse phase medium (RPM) carrier, to provide an osteoconductive & Inductive allograft with ideal handling qualities. OrthoBlast II has enjoyed clinical success in a variety of surgical applications including periarticular defects and long-bone defects. An independent study of two DBM allografts, in cases of metaphyseal and periarticular fractures, concluded that the OrthoBlast II success rate was over 30% higher than the alternative allograft product. In another independent study of the use of DBM allograft products in ankle/hindfoot fusion, 14% of patients with a glycerol based allograft developed a non-union, versus only 8% for the OrthoBlast patients. We offer a 24 hour delivery service for this product. -
 The MediExpand is a one or multi-segmental corpectomy device designed to reconstruct the cervical spine. The implant has an adjustable size increase system which can be adapted to suit the anatomical condition of the patient. Its innovative design reduces the risk of sinking adjacent vertebral bodies and secures anchoring in the adjoining endplates. Featuring an open design the mediExpand offers excellent visibility of the dura during insertion and provides direct access areas for freshening unaffected endplate areas. The large surface area is ideal for bony fusion and offers optimal space for filling up with spongiosa, whilst following the 'guide-rail' principle. The anatomical design provides the reconstruction of the lordotic profile with simple repositioning of the implant by reversing the distraction and allows for individual treatment due to the ability to choose exact height settings.
The MediExpand is a one or multi-segmental corpectomy device designed to reconstruct the cervical spine. The implant has an adjustable size increase system which can be adapted to suit the anatomical condition of the patient. Its innovative design reduces the risk of sinking adjacent vertebral bodies and secures anchoring in the adjoining endplates. Featuring an open design the mediExpand offers excellent visibility of the dura during insertion and provides direct access areas for freshening unaffected endplate areas. The large surface area is ideal for bony fusion and offers optimal space for filling up with spongiosa, whilst following the 'guide-rail' principle. The anatomical design provides the reconstruction of the lordotic profile with simple repositioning of the implant by reversing the distraction and allows for individual treatment due to the ability to choose exact height settings. -
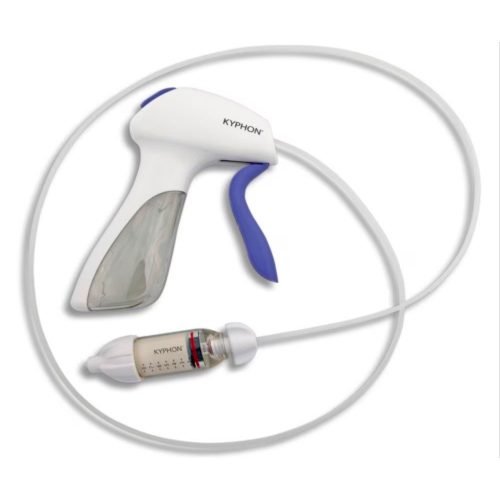
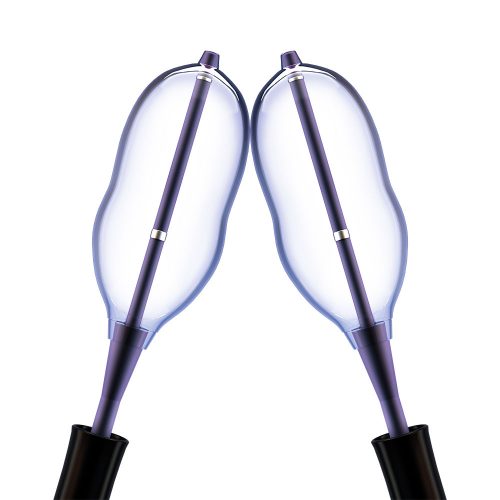
Minimally Invasive Vertebral Compression Fracture (VCF) Treatment
With over 20 years of commitment to procedural options and clinical evidence, Medtronic's balloon kyphoplasty empowers you to treat VCF patients with the unmatched innovations of a reliable, robust product platform. Treating VCF with Balloon Kyphoplasty Balloon kyphoplasty uses orthopedic balloons to restore vertebral height and correct angular deformity from VCF due to osteoporosis, cancer, or benign lesions. After reduction, the balloons are deflated and removed. The resulting cavity allows for a controlled deposition of bone cement to form an internal cast and stabilize the fracture. Key Features of the Kyphon™ Balloon Kyphoplasty Platform- 700 psi max rated inflation pressure, higher than previous 400 psi rated balloons.*
- Cement resistance technique lets you deliver cement through one cannula while the contralateral balloon remains inflated to maintain fracture reduction.
- Kyphon™ cement delivery system (CDS) lets you deliver cement from up to 48 inches away from the radiation source during a kyphoplasty procedure.
-

Minimally Invasive Vertebral Compression Fracture (VCF) Treatment
The Kyphon™ V Premium vertebroplasty platform is designed to facilitate maximum precision of high viscosity cement placement with minimal radiation exposure.1-4 Maximum Precision, Minimum Exposure With the Kyphon™ cement delivery system, the Kyphon V™ premium vertebroplasty system allows you to:1-3- Minimize radiation exposure2 by standing up to four feet away from the radiation source, which has been measured to reduce hand radiation exposure by 70% (compared to Kyphon™ bone filler device when measured with dosimeters under fluoroscopy)*
- Stop cement flow instantly3 by pushing the quick release button to minimize the potential for cement extravasation
* The mean radiation reduction at the hands was 77.79% (p<0.001). Based on an internal testing of 24 total cadaveric procedures (n=12 using Kyphon CDS and n=12 using Kyphon Bone Filler Device). Dosimeters were placed on the wrist and fingers to measure radiation when delivering bone cement into the vertebral body. Radiation result reported is based on adherence to the Directions for Use.
1 Medtronic Data on File – Kyphon V Premium Vertebroplasty 2 Medtronic Data on File – Kyphon Cement Delivery System 3 Medtronic Data on File – Engineering Test Report 4 Medtronic Data on File – Kyphon Kurve
-
 The Jazz™ systems (which includes the Jazz™ Claw Connector) are temporary spinal implants specifically designed to provide a bone anchor for temporary stabilisation during the development of solid bony fusion to assist with the repair of bone fractures. The Jazz™ systems can be used for a number of spinal surgeries:
The Jazz™ systems (which includes the Jazz™ Claw Connector) are temporary spinal implants specifically designed to provide a bone anchor for temporary stabilisation during the development of solid bony fusion to assist with the repair of bone fractures. The Jazz™ systems can be used for a number of spinal surgeries:- Spinal trauma surgery
- Spinal reconstructive surgery
- Spinal degenerative surgery
-

 Integra Mozaik is a collagen ceramic osteoconductive scaffold which is engineered to mimic the composition and structure of natural bone. Mozaik is made up of 80% purified beta-TCP granules & 20% Highly purified type-1 collagen. The TCP provides bone void filling structure whilst the collagen enables a bioactive element inductive to bone growth, an ideal combination. Mozaik is available in two forms in various sizes: Strips & Putties.
Integra Mozaik is a collagen ceramic osteoconductive scaffold which is engineered to mimic the composition and structure of natural bone. Mozaik is made up of 80% purified beta-TCP granules & 20% Highly purified type-1 collagen. The TCP provides bone void filling structure whilst the collagen enables a bioactive element inductive to bone growth, an ideal combination. Mozaik is available in two forms in various sizes: Strips & Putties.Compression Resistant Strip
- Retains bioactive fluids within scaffold to facilitate protein binding.
- Maintains graft volume under compression.
- Bends to conform to uneven surfaces.
Moldable Putty
- Excellent handling and moldability.
- Optimal for placement in irregularly shaped defect sites.






