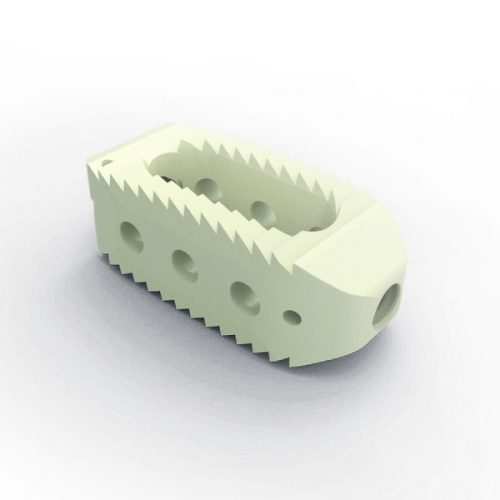
Please feel free to browse our extensive range of Spinal Instruments, Implants and Bone products available to both the NHS and Private Hospital Sector for direct purchase or on a hire basis. A few of our brands include Joimax, Medicon, Spinal Kinetics, Implanet, Edge and Allograft – all in which comply with current traceability legislation and GS1 code practise.
-
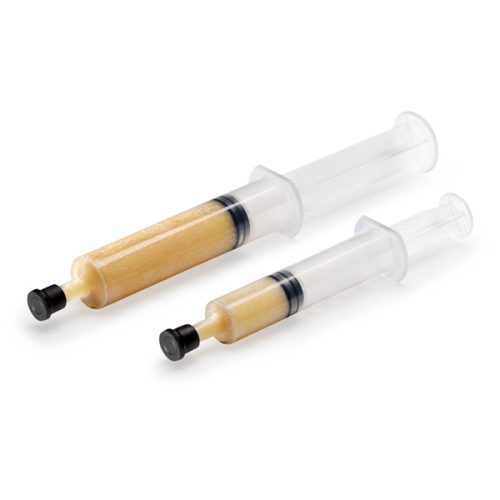 Accell Connexus is highly inductive 2nd Generation DBM with a DBM content of close to 70%. It is sold in a syringe form and also contains the unique RPM carrier which is also an inductive material, giving it exceptional handling characteristics. Accell Connexus should be mixed with any Allograft, Autograft or a synthetic scaffold to provide the ideal recipe for fusion – of conductivity and inductivity. Accell Technology features a patented process that produces the Accell bone matrix (ABM) which is an open-structured, dispersed form of Dynamic Bone Matrix (DBM). The ABM study uses a skeletally mature sheep model with cylindrical 5mm metatarsal defect which is created in tibial diaphysis and which shows excellent results after 4 weeks. This product benefits from validated osteoinductive potential, is mouldable and easy to pack into defects making it ready to use. Case studies have shown positive results when used in:
Accell Connexus is highly inductive 2nd Generation DBM with a DBM content of close to 70%. It is sold in a syringe form and also contains the unique RPM carrier which is also an inductive material, giving it exceptional handling characteristics. Accell Connexus should be mixed with any Allograft, Autograft or a synthetic scaffold to provide the ideal recipe for fusion – of conductivity and inductivity. Accell Technology features a patented process that produces the Accell bone matrix (ABM) which is an open-structured, dispersed form of Dynamic Bone Matrix (DBM). The ABM study uses a skeletally mature sheep model with cylindrical 5mm metatarsal defect which is created in tibial diaphysis and which shows excellent results after 4 weeks. This product benefits from validated osteoinductive potential, is mouldable and easy to pack into defects making it ready to use. Case studies have shown positive results when used in:- Spine: Intertransverse process fusion
- Hand: Cystic scaphoid nonunion
- Foot & Ankle: - Tibial osteotomy - Hindfoot and ankle nonunions
Accell Connexus Varients
Connexus Putty 0.5cc - Syringe Connexus Putty 1cc – Syringe Connexus Putty 5cc – Syringe Connexus Putty 10cc – Syringe We use a guaranteed 24 hour delivery service for this product. -
 Allograft bone is an ideal alternative to autograft for quick, complete bone regeneration. Cancellous chips and granules form the Osteoconductive scaffold for new bone formation while maintaining the porosity essential for tissue and vascular regrowth. Cancellous Bone packs well into any size or shape defect, for maximum surgical flexibility and is available for immediate use with no refrigeration or thawing required. Potential complications of iliac crest bone harvesting, such as morbidity, limited supply, and pain, make Cancellous bone an ideal autograft substitute which has been obtained from an AATB-Accredited Tissue Bank, ensuring the highest quality bone through rigorous donor screening, testing and sterilisation. In many cases, cancellous bone is preferable to cortical bone due to its higher surface area and open porous structure that allows for easy cellular penetration and incorporation and is a perfect compliment to IsoTis’ Accell® product line, and can be used as a bone graft extender or for composite grafting. We supply Allograft Cancellous Bone in a variety of sizes which can be purchased in chip or crushed formation - please see the tab below for further information. We use a guaranteed 24 hour delivery service for this product.
Allograft bone is an ideal alternative to autograft for quick, complete bone regeneration. Cancellous chips and granules form the Osteoconductive scaffold for new bone formation while maintaining the porosity essential for tissue and vascular regrowth. Cancellous Bone packs well into any size or shape defect, for maximum surgical flexibility and is available for immediate use with no refrigeration or thawing required. Potential complications of iliac crest bone harvesting, such as morbidity, limited supply, and pain, make Cancellous bone an ideal autograft substitute which has been obtained from an AATB-Accredited Tissue Bank, ensuring the highest quality bone through rigorous donor screening, testing and sterilisation. In many cases, cancellous bone is preferable to cortical bone due to its higher surface area and open porous structure that allows for easy cellular penetration and incorporation and is a perfect compliment to IsoTis’ Accell® product line, and can be used as a bone graft extender or for composite grafting. We supply Allograft Cancellous Bone in a variety of sizes which can be purchased in chip or crushed formation - please see the tab below for further information. We use a guaranteed 24 hour delivery service for this product. -

 Ballast is an easy-to-deliver, inductive bone graft used for posterior spinal fusion procedures. It is designed to contour and maximise contact with the anatomy whilst still maintaining shape and volume under compression. Key features:
Ballast is an easy-to-deliver, inductive bone graft used for posterior spinal fusion procedures. It is designed to contour and maximise contact with the anatomy whilst still maintaining shape and volume under compression. Key features:- Delivers a large, consistent volume of graft (6-9cc per level per side)
- Withstands compressive forces of the Paraspinal Muscles and does not flatten or displace
- Maximises Osteoinductive potential with 100% DBM, within the mesh pouch, in a challenging application
- Contours around spinal hardware and anatomy
- Simple to deliver and position in the Posterolateral Gutters
-
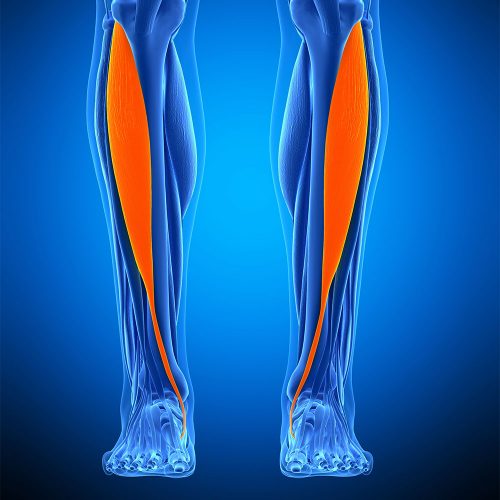 The Anterior Tibialis is an incredibly strong tendon responsible for flexion in the foot. In regards to their use as allografts, our Frozen Anterior Tibialis Tendon is most commonly used for tendon reconstruction procedures including the ACL (Anterior Cruciate Ligament) and UCL (Ulnar Collateral Ligament). Less commonly, it can also be used for ankle stabilisation, ligament repair, joint, and bicep ruptures. Recent research has found the use of freeze-dried tibialis anterior allografts achieved 'excellent to good clinical outcomes' (according to Tegner-Lysholm grading scale) when used for tibial fixation in ACL reconstruction and revision ACL surgeries. Prior to this, four-strand hamstring autograft was most commonly used for ACL reconstruction, however the research found that upon providing patients with the information surrounding additional benefits of using tibialis anterior allografts - the majority of patients chose the allograft option. Further information can be found on the published research findings here. Alongside them being preferred by patients, there are multiple benefits for Physicians using the Frozen Anterior Tibialis Tendon in ACL reconstruction, including but not limited to; Allowing the Physician to predetermine the diameter size of tunnels, predetermine graft length for the procedure and allowing for various fixation techniques.
The Anterior Tibialis is an incredibly strong tendon responsible for flexion in the foot. In regards to their use as allografts, our Frozen Anterior Tibialis Tendon is most commonly used for tendon reconstruction procedures including the ACL (Anterior Cruciate Ligament) and UCL (Ulnar Collateral Ligament). Less commonly, it can also be used for ankle stabilisation, ligament repair, joint, and bicep ruptures. Recent research has found the use of freeze-dried tibialis anterior allografts achieved 'excellent to good clinical outcomes' (according to Tegner-Lysholm grading scale) when used for tibial fixation in ACL reconstruction and revision ACL surgeries. Prior to this, four-strand hamstring autograft was most commonly used for ACL reconstruction, however the research found that upon providing patients with the information surrounding additional benefits of using tibialis anterior allografts - the majority of patients chose the allograft option. Further information can be found on the published research findings here. Alongside them being preferred by patients, there are multiple benefits for Physicians using the Frozen Anterior Tibialis Tendon in ACL reconstruction, including but not limited to; Allowing the Physician to predetermine the diameter size of tunnels, predetermine graft length for the procedure and allowing for various fixation techniques. -
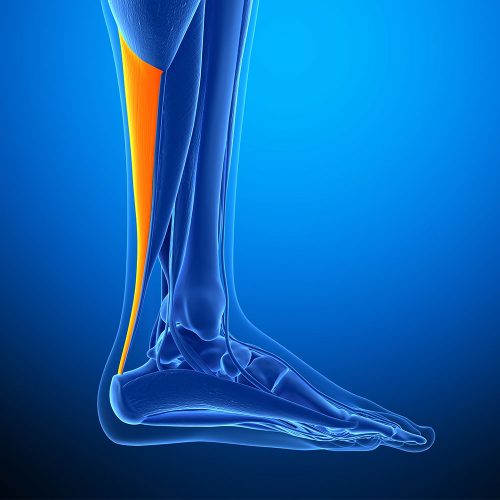 Although part of the hamstring, the Semitendinosus Tendon is commonly used for Ankle Stabilisation and Achilles Tendon Repair. We supply a high-quality frozen and professionally sterilised Semi-T Achilles Tendon for use in surgical operations to repair the Achilles Tendon. In particular for chronic tears of the Achilles tendon, when there is a gap of more than 5cm, it is most usually recommended that a primary tendon reconstruction via transfer of an allograft is undertaken. This is used in order to replace the missing tissue, due to the lack of healthy tissue in order to perform the repair directly. The surgical process involves drilling a tunnel into the base of the calcaneus and the harvested tendon is then fed through the tunnel and both stitched and screwed in place.
Although part of the hamstring, the Semitendinosus Tendon is commonly used for Ankle Stabilisation and Achilles Tendon Repair. We supply a high-quality frozen and professionally sterilised Semi-T Achilles Tendon for use in surgical operations to repair the Achilles Tendon. In particular for chronic tears of the Achilles tendon, when there is a gap of more than 5cm, it is most usually recommended that a primary tendon reconstruction via transfer of an allograft is undertaken. This is used in order to replace the missing tissue, due to the lack of healthy tissue in order to perform the repair directly. The surgical process involves drilling a tunnel into the base of the calcaneus and the harvested tendon is then fed through the tunnel and both stitched and screwed in place. -
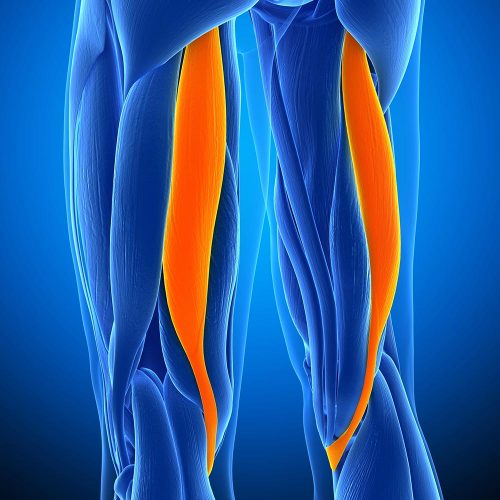 The frozen Semi-T (Semitendinosus) Double Strand Tendon provides surgeons with the ability to reconstruct a torn ligament - the Semitendinosus in particular, is commonly used for ankle stabilisation. A very common occurrence for patients who have suffered a traumatic ligament tear is to experience laxity, which can heavily impede on their ability to remain active. The unique nature of using a frozen double strand tendon during reconstruction of cruciate ligaments, can help considerably with repairing and stabilising the ligament, and is why the double strand is so effective in the reconstruction of torn ligaments. There are numerous benefits of using our Frozen Semi-T Double Strand Tendons for reconstruction surgery, in particular, eliminating the need of harvesting autograft, thus removing additional pain and surgical scars, as well as providing the surgeon with the best fixation technique - to name a few.
The frozen Semi-T (Semitendinosus) Double Strand Tendon provides surgeons with the ability to reconstruct a torn ligament - the Semitendinosus in particular, is commonly used for ankle stabilisation. A very common occurrence for patients who have suffered a traumatic ligament tear is to experience laxity, which can heavily impede on their ability to remain active. The unique nature of using a frozen double strand tendon during reconstruction of cruciate ligaments, can help considerably with repairing and stabilising the ligament, and is why the double strand is so effective in the reconstruction of torn ligaments. There are numerous benefits of using our Frozen Semi-T Double Strand Tendons for reconstruction surgery, in particular, eliminating the need of harvesting autograft, thus removing additional pain and surgical scars, as well as providing the surgeon with the best fixation technique - to name a few. -

 Integra Mozaik is a collagen ceramic osteoconductive scaffold which is engineered to mimic the composition and structure of natural bone. Mozaik is made up of 80% purified beta-TCP granules & 20% Highly purified type-1 collagen. The TCP provides bone void filling structure whilst the collagen enables a bioactive element inductive to bone growth, an ideal combination. Mozaik is available in two forms in various sizes: Strips & Putties.
Integra Mozaik is a collagen ceramic osteoconductive scaffold which is engineered to mimic the composition and structure of natural bone. Mozaik is made up of 80% purified beta-TCP granules & 20% Highly purified type-1 collagen. The TCP provides bone void filling structure whilst the collagen enables a bioactive element inductive to bone growth, an ideal combination. Mozaik is available in two forms in various sizes: Strips & Putties.Compression Resistant Strip
- Retains bioactive fluids within scaffold to facilitate protein binding.
- Maintains graft volume under compression.
- Bends to conform to uneven surfaces.
Moldable Putty
- Excellent handling and moldability.
- Optimal for placement in irregularly shaped defect sites.
-
 The Jazz™ systems (which includes the Jazz™ Claw Connector) are temporary spinal implants specifically designed to provide a bone anchor for temporary stabilisation during the development of solid bony fusion to assist with the repair of bone fractures. The Jazz™ systems can be used for a number of spinal surgeries:
The Jazz™ systems (which includes the Jazz™ Claw Connector) are temporary spinal implants specifically designed to provide a bone anchor for temporary stabilisation during the development of solid bony fusion to assist with the repair of bone fractures. The Jazz™ systems can be used for a number of spinal surgeries:- Spinal trauma surgery
- Spinal reconstructive surgery
- Spinal degenerative surgery
-

Minimally Invasive Vertebral Compression Fracture (VCF) Treatment
The Kyphon™ V Premium vertebroplasty platform is designed to facilitate maximum precision of high viscosity cement placement with minimal radiation exposure.1-4 Maximum Precision, Minimum Exposure With the Kyphon™ cement delivery system, the Kyphon V™ premium vertebroplasty system allows you to:1-3- Minimize radiation exposure2 by standing up to four feet away from the radiation source, which has been measured to reduce hand radiation exposure by 70% (compared to Kyphon™ bone filler device when measured with dosimeters under fluoroscopy)*
- Stop cement flow instantly3 by pushing the quick release button to minimize the potential for cement extravasation
* The mean radiation reduction at the hands was 77.79% (p<0.001). Based on an internal testing of 24 total cadaveric procedures (n=12 using Kyphon CDS and n=12 using Kyphon Bone Filler Device). Dosimeters were placed on the wrist and fingers to measure radiation when delivering bone cement into the vertebral body. Radiation result reported is based on adherence to the Directions for Use.
1 Medtronic Data on File – Kyphon V Premium Vertebroplasty 2 Medtronic Data on File – Kyphon Cement Delivery System 3 Medtronic Data on File – Engineering Test Report 4 Medtronic Data on File – Kyphon Kurve
-
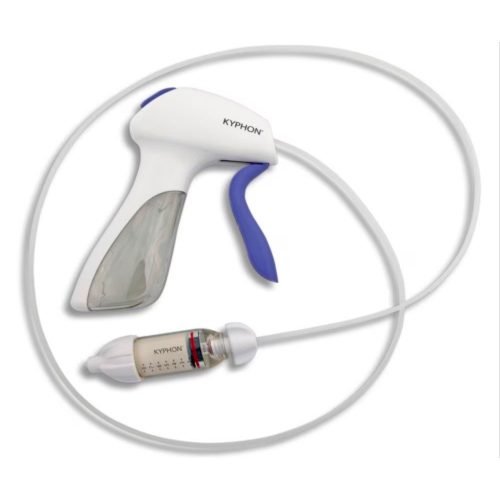
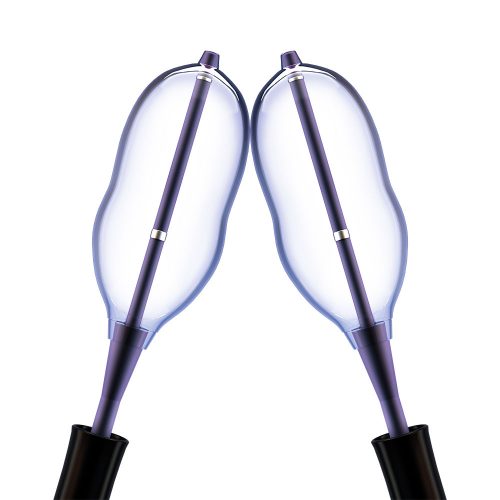
Minimally Invasive Vertebral Compression Fracture (VCF) Treatment
With over 20 years of commitment to procedural options and clinical evidence, Medtronic's balloon kyphoplasty empowers you to treat VCF patients with the unmatched innovations of a reliable, robust product platform. Treating VCF with Balloon Kyphoplasty Balloon kyphoplasty uses orthopedic balloons to restore vertebral height and correct angular deformity from VCF due to osteoporosis, cancer, or benign lesions. After reduction, the balloons are deflated and removed. The resulting cavity allows for a controlled deposition of bone cement to form an internal cast and stabilize the fracture. Key Features of the Kyphon™ Balloon Kyphoplasty Platform- 700 psi max rated inflation pressure, higher than previous 400 psi rated balloons.*
- Cement resistance technique lets you deliver cement through one cannula while the contralateral balloon remains inflated to maintain fracture reduction.
- Kyphon™ cement delivery system (CDS) lets you deliver cement from up to 48 inches away from the radiation source during a kyphoplasty procedure.