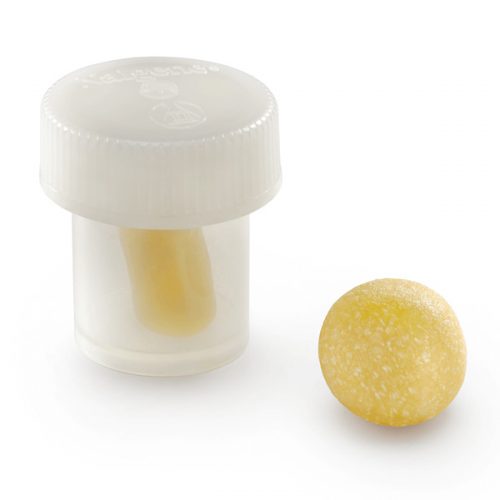
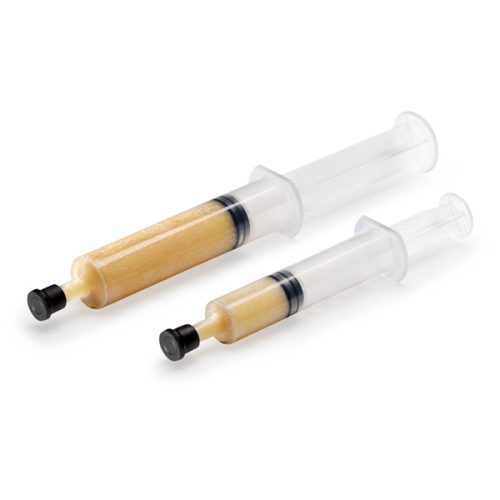
Orthoblast II is a powerful choice of bone grafting and it is what is termed a “complete” bone graft, offering both conductivity and inductivity to the user Surgeon. It is available as a Putty in a tub where the conductive bone graft granules mixed in with the DBM Putty are larger given the method of delivery. This is a crunchy graft when handled by the Surgeon. The other method of delivery with this product is via a syringe, where the conductive granules are slightly smaller simply because they need to go through the tip of the syringe. This is no less a complete bone graft however, it is simply another method of delivery for the Surgeon, and contains demineralized bone matrix (DBM) to stimulate and regenerate new bone growth. It is a highly inductive material supplied in syringe form (smaller particulate bone granules) and in tub form (larger particulate bone granules).
OrthoBlast® II therefore, combines demineralized allograft bone with cancellous bone in a reverse phase medium (RPM) carrier, to provide an osteoconductive & Inductive allograft with ideal handling qualities.
OrthoBlast II has enjoyed clinical success in a variety of surgical applications including periarticular defects and long-bone defects. An independent study of two DBM allografts, in cases of metaphyseal and periarticular fractures, concluded that the OrthoBlast II success rate was over 30% higher than the alternative allograft product. In another independent study of the use of DBM allograft products in ankle/hindfoot fusion, 14% of patients with a glycerol based allograft developed a non-union, versus only 8% for the OrthoBlast patients.
We offer a 24 hour delivery service for this product.