Please feel free to browse our extensive range of Spinal Instruments, Implants and Bone products available to both the NHS and Private Hospital Sector for direct purchase or on a hire basis. A few of our brands include Joimax, Medicon, Spinal Kinetics, Implanet, Edge and Allograft – all in which comply with current traceability legislation and GS1 code practise.
-

 Ballast is an easy-to-deliver, inductive bone graft used for posterior spinal fusion procedures. It is designed to contour and maximise contact with the anatomy whilst still maintaining shape and volume under compression. Key features:
Ballast is an easy-to-deliver, inductive bone graft used for posterior spinal fusion procedures. It is designed to contour and maximise contact with the anatomy whilst still maintaining shape and volume under compression. Key features:- Delivers a large, consistent volume of graft (6-9cc per level per side)
- Withstands compressive forces of the Paraspinal Muscles and does not flatten or displace
- Maximises Osteoinductive potential with 100% DBM, within the mesh pouch, in a challenging application
- Contours around spinal hardware and anatomy
- Simple to deliver and position in the Posterolateral Gutters
-
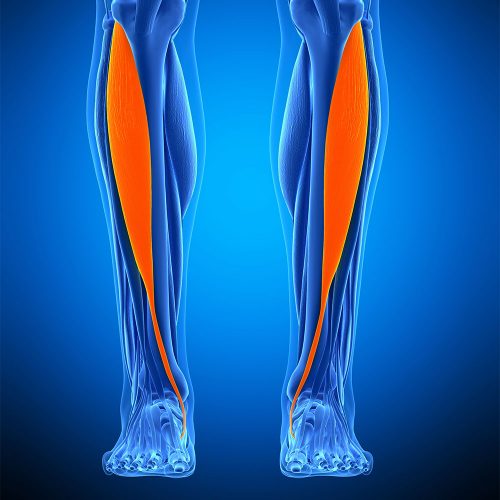 The Anterior Tibialis is an incredibly strong tendon responsible for flexion in the foot. In regards to their use as allografts, our Frozen Anterior Tibialis Tendon is most commonly used for tendon reconstruction procedures including the ACL (Anterior Cruciate Ligament) and UCL (Ulnar Collateral Ligament). Less commonly, it can also be used for ankle stabilisation, ligament repair, joint, and bicep ruptures. Recent research has found the use of freeze-dried tibialis anterior allografts achieved 'excellent to good clinical outcomes' (according to Tegner-Lysholm grading scale) when used for tibial fixation in ACL reconstruction and revision ACL surgeries. Prior to this, four-strand hamstring autograft was most commonly used for ACL reconstruction, however the research found that upon providing patients with the information surrounding additional benefits of using tibialis anterior allografts - the majority of patients chose the allograft option. Further information can be found on the published research findings here. Alongside them being preferred by patients, there are multiple benefits for Physicians using the Frozen Anterior Tibialis Tendon in ACL reconstruction, including but not limited to; Allowing the Physician to predetermine the diameter size of tunnels, predetermine graft length for the procedure and allowing for various fixation techniques.
The Anterior Tibialis is an incredibly strong tendon responsible for flexion in the foot. In regards to their use as allografts, our Frozen Anterior Tibialis Tendon is most commonly used for tendon reconstruction procedures including the ACL (Anterior Cruciate Ligament) and UCL (Ulnar Collateral Ligament). Less commonly, it can also be used for ankle stabilisation, ligament repair, joint, and bicep ruptures. Recent research has found the use of freeze-dried tibialis anterior allografts achieved 'excellent to good clinical outcomes' (according to Tegner-Lysholm grading scale) when used for tibial fixation in ACL reconstruction and revision ACL surgeries. Prior to this, four-strand hamstring autograft was most commonly used for ACL reconstruction, however the research found that upon providing patients with the information surrounding additional benefits of using tibialis anterior allografts - the majority of patients chose the allograft option. Further information can be found on the published research findings here. Alongside them being preferred by patients, there are multiple benefits for Physicians using the Frozen Anterior Tibialis Tendon in ACL reconstruction, including but not limited to; Allowing the Physician to predetermine the diameter size of tunnels, predetermine graft length for the procedure and allowing for various fixation techniques. -
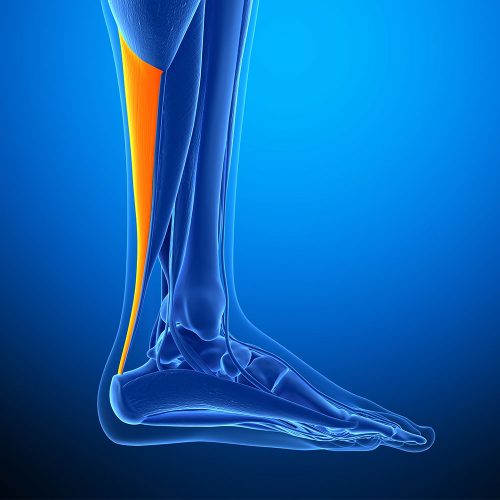 Although part of the hamstring, the Semitendinosus Tendon is commonly used for Ankle Stabilisation and Achilles Tendon Repair. We supply a high-quality frozen and professionally sterilised Semi-T Achilles Tendon for use in surgical operations to repair the Achilles Tendon. In particular for chronic tears of the Achilles tendon, when there is a gap of more than 5cm, it is most usually recommended that a primary tendon reconstruction via transfer of an allograft is undertaken. This is used in order to replace the missing tissue, due to the lack of healthy tissue in order to perform the repair directly. The surgical process involves drilling a tunnel into the base of the calcaneus and the harvested tendon is then fed through the tunnel and both stitched and screwed in place.
Although part of the hamstring, the Semitendinosus Tendon is commonly used for Ankle Stabilisation and Achilles Tendon Repair. We supply a high-quality frozen and professionally sterilised Semi-T Achilles Tendon for use in surgical operations to repair the Achilles Tendon. In particular for chronic tears of the Achilles tendon, when there is a gap of more than 5cm, it is most usually recommended that a primary tendon reconstruction via transfer of an allograft is undertaken. This is used in order to replace the missing tissue, due to the lack of healthy tissue in order to perform the repair directly. The surgical process involves drilling a tunnel into the base of the calcaneus and the harvested tendon is then fed through the tunnel and both stitched and screwed in place. -
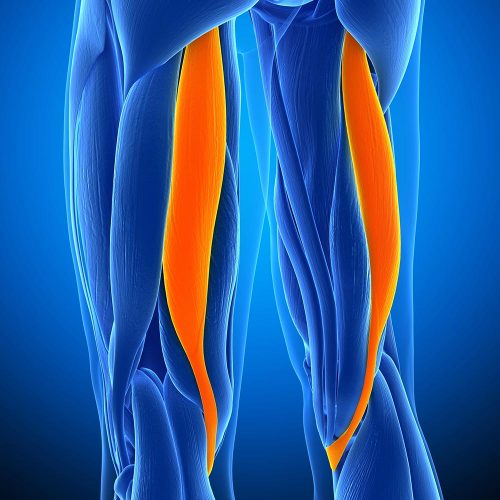 The frozen Semi-T (Semitendinosus) Double Strand Tendon provides surgeons with the ability to reconstruct a torn ligament - the Semitendinosus in particular, is commonly used for ankle stabilisation. A very common occurrence for patients who have suffered a traumatic ligament tear is to experience laxity, which can heavily impede on their ability to remain active. The unique nature of using a frozen double strand tendon during reconstruction of cruciate ligaments, can help considerably with repairing and stabilising the ligament, and is why the double strand is so effective in the reconstruction of torn ligaments. There are numerous benefits of using our Frozen Semi-T Double Strand Tendons for reconstruction surgery, in particular, eliminating the need of harvesting autograft, thus removing additional pain and surgical scars, as well as providing the surgeon with the best fixation technique - to name a few.
The frozen Semi-T (Semitendinosus) Double Strand Tendon provides surgeons with the ability to reconstruct a torn ligament - the Semitendinosus in particular, is commonly used for ankle stabilisation. A very common occurrence for patients who have suffered a traumatic ligament tear is to experience laxity, which can heavily impede on their ability to remain active. The unique nature of using a frozen double strand tendon during reconstruction of cruciate ligaments, can help considerably with repairing and stabilising the ligament, and is why the double strand is so effective in the reconstruction of torn ligaments. There are numerous benefits of using our Frozen Semi-T Double Strand Tendons for reconstruction surgery, in particular, eliminating the need of harvesting autograft, thus removing additional pain and surgical scars, as well as providing the surgeon with the best fixation technique - to name a few. -

Minimally Invasive Vertebral Compression Fracture (VCF) Treatment
The Kyphon™ V Premium vertebroplasty platform is designed to facilitate maximum precision of high viscosity cement placement with minimal radiation exposure.1-4 Maximum Precision, Minimum Exposure With the Kyphon™ cement delivery system, the Kyphon V™ premium vertebroplasty system allows you to:1-3- Minimize radiation exposure2 by standing up to four feet away from the radiation source, which has been measured to reduce hand radiation exposure by 70% (compared to Kyphon™ bone filler device when measured with dosimeters under fluoroscopy)*
- Stop cement flow instantly3 by pushing the quick release button to minimize the potential for cement extravasation
* The mean radiation reduction at the hands was 77.79% (p<0.001). Based on an internal testing of 24 total cadaveric procedures (n=12 using Kyphon CDS and n=12 using Kyphon Bone Filler Device). Dosimeters were placed on the wrist and fingers to measure radiation when delivering bone cement into the vertebral body. Radiation result reported is based on adherence to the Directions for Use.
1 Medtronic Data on File – Kyphon V Premium Vertebroplasty 2 Medtronic Data on File – Kyphon Cement Delivery System 3 Medtronic Data on File – Engineering Test Report 4 Medtronic Data on File – Kyphon Kurve
-
Minimally Invasive Vertebral Compression Fracture (VCF) Treatment
With over 20 years of commitment to procedural options and clinical evidence, Medtronic's balloon kyphoplasty empowers you to treat VCF patients with the unmatched innovations of a reliable, robust product platform. Treating VCF with Balloon Kyphoplasty Balloon kyphoplasty uses orthopedic balloons to restore vertebral height and correct angular deformity from VCF due to osteoporosis, cancer, or benign lesions. After reduction, the balloons are deflated and removed. The resulting cavity allows for a controlled deposition of bone cement to form an internal cast and stabilize the fracture. Key Features of the Kyphon™ Balloon Kyphoplasty Platform- 700 psi max rated inflation pressure, higher than previous 400 psi rated balloons.*
- Cement resistance technique lets you deliver cement through one cannula while the contralateral balloon remains inflated to maintain fracture reduction.
- Kyphon™ cement delivery system (CDS) lets you deliver cement from up to 48 inches away from the radiation source during a kyphoplasty procedure.
-
 The MediExpand is a one or multi-segmental corpectomy device designed to reconstruct the cervical spine. The implant has an adjustable size increase system which can be adapted to suit the anatomical condition of the patient. Its innovative design reduces the risk of sinking adjacent vertebral bodies and secures anchoring in the adjoining endplates. Featuring an open design the mediExpand offers excellent visibility of the dura during insertion and provides direct access areas for freshening unaffected endplate areas. The large surface area is ideal for bony fusion and offers optimal space for filling up with spongiosa, whilst following the 'guide-rail' principle. The anatomical design provides the reconstruction of the lordotic profile with simple repositioning of the implant by reversing the distraction and allows for individual treatment due to the ability to choose exact height settings.
The MediExpand is a one or multi-segmental corpectomy device designed to reconstruct the cervical spine. The implant has an adjustable size increase system which can be adapted to suit the anatomical condition of the patient. Its innovative design reduces the risk of sinking adjacent vertebral bodies and secures anchoring in the adjoining endplates. Featuring an open design the mediExpand offers excellent visibility of the dura during insertion and provides direct access areas for freshening unaffected endplate areas. The large surface area is ideal for bony fusion and offers optimal space for filling up with spongiosa, whilst following the 'guide-rail' principle. The anatomical design provides the reconstruction of the lordotic profile with simple repositioning of the implant by reversing the distraction and allows for individual treatment due to the ability to choose exact height settings. -

For painful bone tumours
The OsteoCoolTM RF Ablation System is cooled radiofrequency (RF) ablation technology. It offers simultaneous, dual-probe capabilities for treating painful bone tumours. Product DetailsThe OsteoCool RF Ablation system is cooled radiofrequency (RF) ablation technology. It offers simultaneous, dual-probe capabilities for treating painful bone tumours.
Coaxial, bipolar technology delivers RF energy to the site, and automatically moderates power to keep RF heating within the desired treatment range. This reduces risk of potential thermal damage to adjacent tissue.1
The active tip of the ablation probe is internally cooled with circulating water. RF energy heats the tissue while circulating water moderates the temperature close to the active tip. This combination:
- Creates large volume lesions without excessive heating at the active tip
- Minimises potential for char
The OsteoCool RF ablation probes are sterile and intended for single use.
1 Based on internal testing. Data on file.
-
 The Seaspine Strand is made from 100% demineralised bone fibres and is designed to encourage bone growth. Developed through a disciplined R&D procedure, the Strand has evaluated a variety of fibre geometries to deliver osteoinductivity, osteoconductivity, intraoperative handling, and controlled expansion. The Seaspine Strand Plus is powered by Accell® Bone Matrix (ABM) which provides both immediate and sustained release of growth factors. Key Features for the Strand:
The Seaspine Strand is made from 100% demineralised bone fibres and is designed to encourage bone growth. Developed through a disciplined R&D procedure, the Strand has evaluated a variety of fibre geometries to deliver osteoinductivity, osteoconductivity, intraoperative handling, and controlled expansion. The Seaspine Strand Plus is powered by Accell® Bone Matrix (ABM) which provides both immediate and sustained release of growth factors. Key Features for the Strand:- 100% Demineralised Bone Fibres
- Expands to Fill Gaps
- Cellular Highways
- Simplicity of Hydration
- 100% Demineralised Bone Fibres with ABM
- 100% Demineralised Bone Fibres
- Expands to Fill Gaps
- Cellular Highways
- Simplicity of Hydration
-
 We supply a wide range of articulated joint repair ligaments and tendons including:All of our tendons and ligaments undergo a rigorous Supercritical CO2 Sterilisation Process which is explained in further detail below.
We supply a wide range of articulated joint repair ligaments and tendons including:All of our tendons and ligaments undergo a rigorous Supercritical CO2 Sterilisation Process which is explained in further detail below.- Patella Tendon Whole
- Patella Tendon Hemi
- Pre-Shaped Patella Tendon
- Achilles Tendon w/Bone Block
- Achilles Tendon w/out Bone Block
- Pre-Shaped Achilles Tendon w/Bone Block
- Gracilis Tendon
- Semitendinosus Tendon
- Semitendinosus-Gracilis Tendon
- Tibialis Tendon Anterior
- Tibialis Tendon Posterior
- Peroneus Longus




