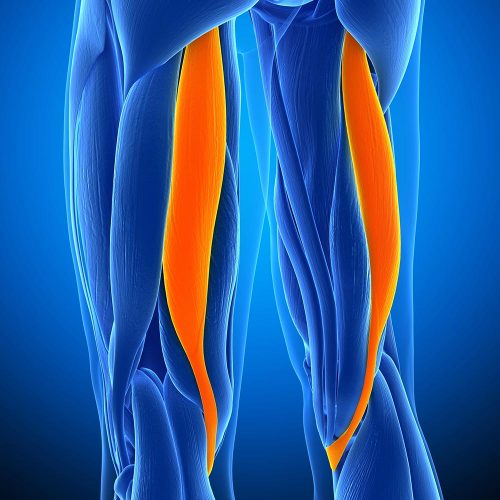
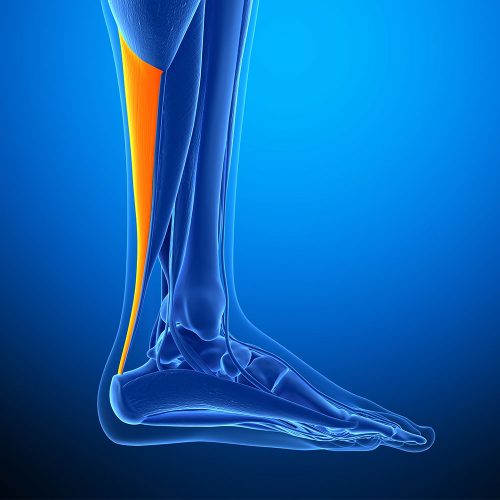
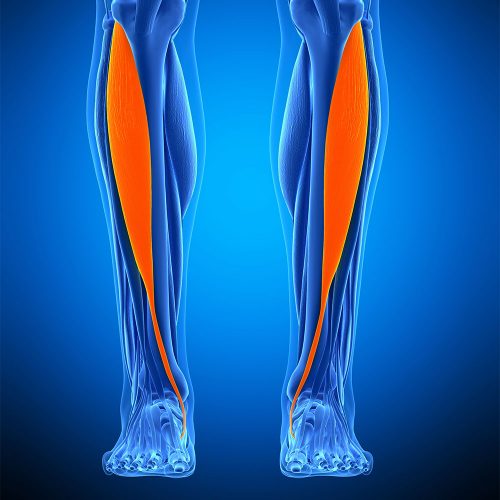
The Anterior Tibialis is an incredibly strong tendon responsible for flexion in the foot. In regards to their use as allografts, our Frozen Anterior Tibialis Tendon is most commonly used for tendon reconstruction procedures including the ACL (Anterior Cruciate Ligament) and UCL (Ulnar Collateral Ligament). Less commonly, it can also be used for ankle stabilisation, ligament repair, joint, and bicep ruptures.
Recent research has found the use of freeze-dried tibialis anterior allografts achieved 'excellent to good clinical outcomes' (according to Tegner-Lysholm grading scale) when used for tibial fixation in ACL reconstruction and revision ACL surgeries. Prior to this, four-strand hamstring autograft was most commonly used for ACL reconstruction, however the research found that upon providing patients with the information surrounding additional benefits of using tibialis anterior allografts - the majority of patients chose the allograft option. Further information can be found on the
published research findings here.
Alongside them being preferred by patients, there are multiple benefits for Physicians using the Frozen Anterior Tibialis Tendon in ACL reconstruction, including but not limited to; Allowing the Physician to predetermine the diameter size of tunnels, predetermine graft length for the procedure and allowing for various fixation techniques.